The basics
Elimination reactions are a type of organic transformation during which two σ (single) bonds are broken and a new π (double) bond is formed. A classical elimination reaction involves the loss of one hydrogen and one leaving groups on two adjacent carbons under the action of a base. One such elimination is the reaction of fluoride (F–, a strong base) with chloroethane, yielding ethene, HF, and a chloride anion:

Elimination reactions can occur via a variety of mechanisms, one of which is the E2 mechanism, which is a concerted (one-step) reaction. During an E2 mechanism, the base removes the hydrogen while a simultaneous cleavage of the bond to the leaving group allows for a new double bond to form. The elimination from chloroethane with fluoride happens through an E2 mechanism. We represent the mechanism with the three arrows below:
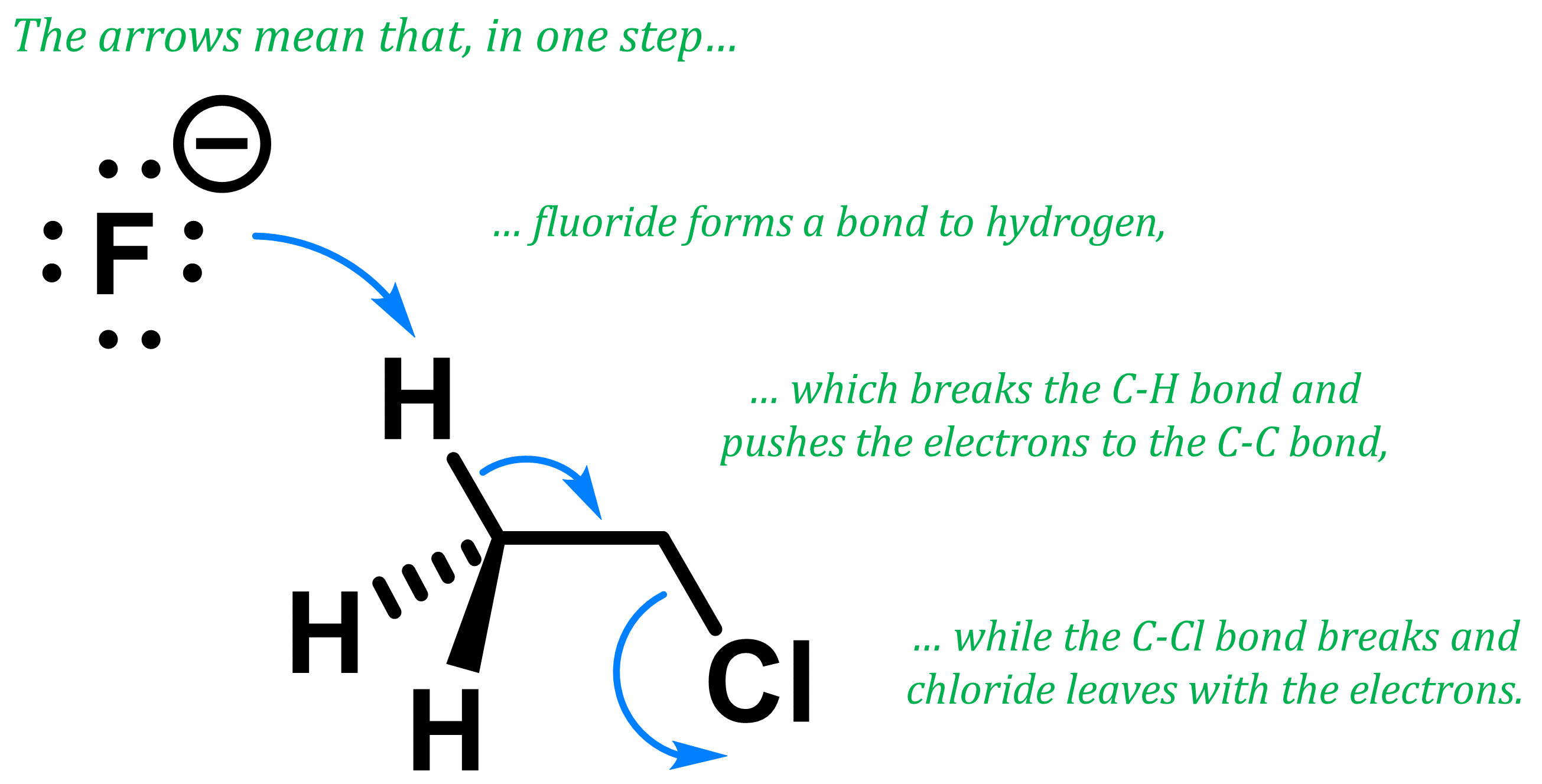
So how does it look like when fluoride reacts with chloroethane in an E2 mechanism?
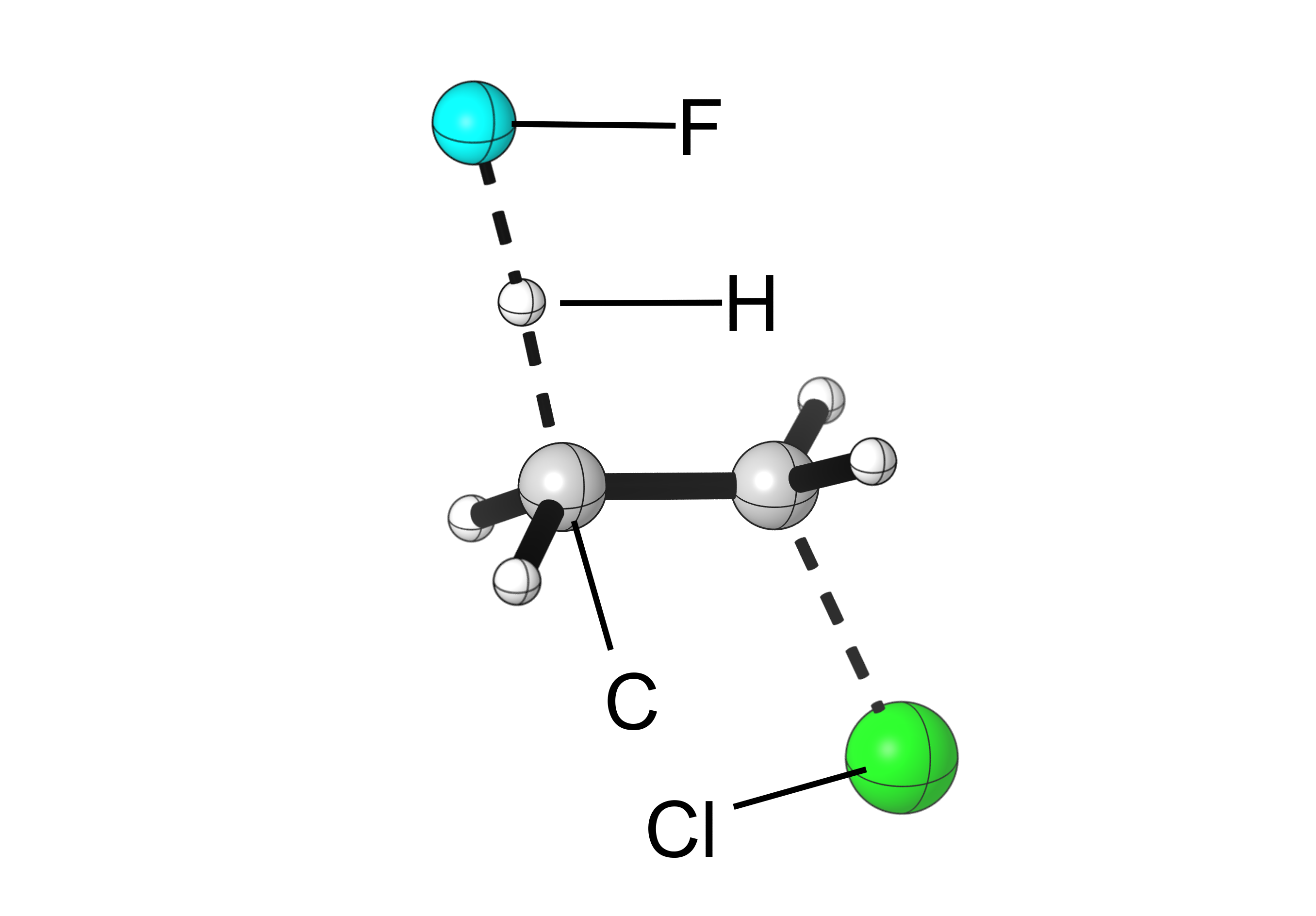
A few points to notice:
- All bond formations and cleavages happen simultaneously.
- The central carbon atoms rehybridize from sp3 (tetrahedral) to sp2 (trigonal planar) as the C-C bond becomes a C=C bond. Also, notice how that C-C bond becomes shorter as it becomes a double bond.
- The hydrogen and leaving group are antiperiplanar, meaning they are on opposite faces of the forming alkene. This is a requirement for most concerted E2 reactions.
Chloroethane elimination by phosphide
A similar E2 reaction can happen using phosphide (PH2–) as a base instead:


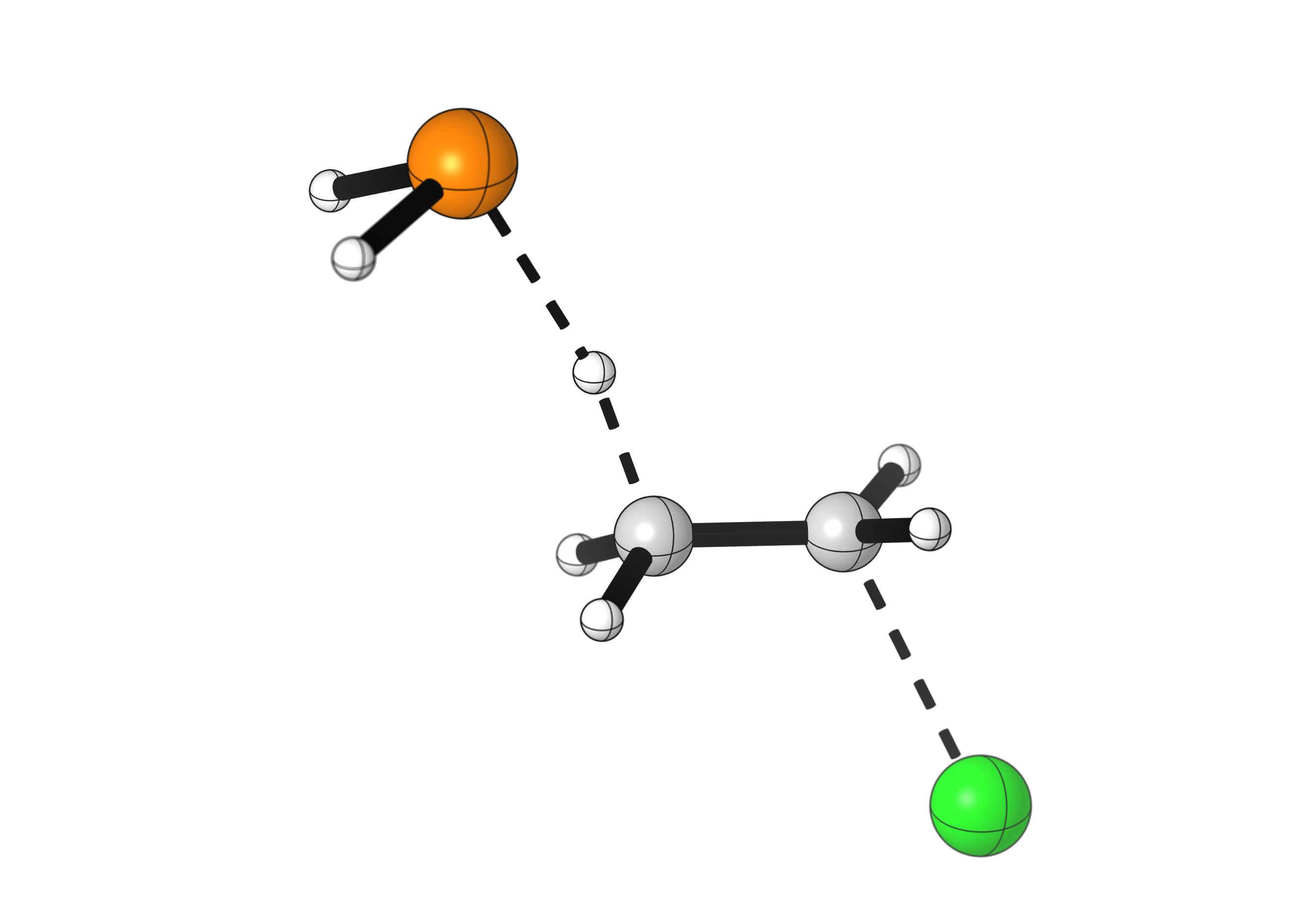
Phosphide is a weaker base than fluoride, and a much stronger nucleophile, so it prefers to react with chloroethane via a nucleophilic substitution mechanism (SN2).
Other mechanisms for eliminations on alkyl electrophiles include the E1 and E1cb mechanisms, both of which involve two steps and an intermediate.
Download the data
- Intrinsic reaction coordinate (IRC) file for the elimination from chloroethane by fluoride (.xyz)
- IRC file for the elimination from chloroethane by phosphide (.xyz)
Animations created by Joie Kelly, Rebecca Zaki, Sean Larmore, and Floris Buttard.
