The reaction of alkenes with boranes (compounds with a B-H bond) is called hydroboration. The product of that reaction is an alkyl borane, which can be transformed to an alcohol by an oxidation reaction. The overall process is a good method to synthesize anti-Markovnikov (terminal) alcohols from terminal alkenes.
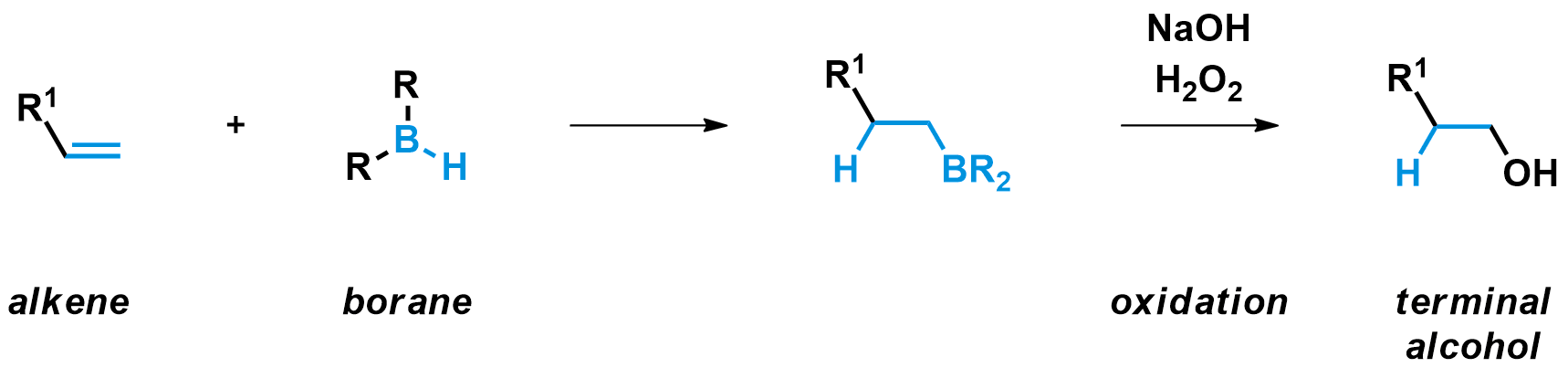
Hydroboration
The first step of that process is the hydroboration. During this reaction, the borane approaches the alkene until its B-H bond breaks, forming new bonds between the alkene carbons and the H and B atoms. One of the simplest system the reaction of BH3 with propene, as shown below.
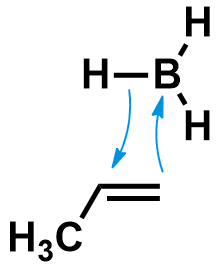
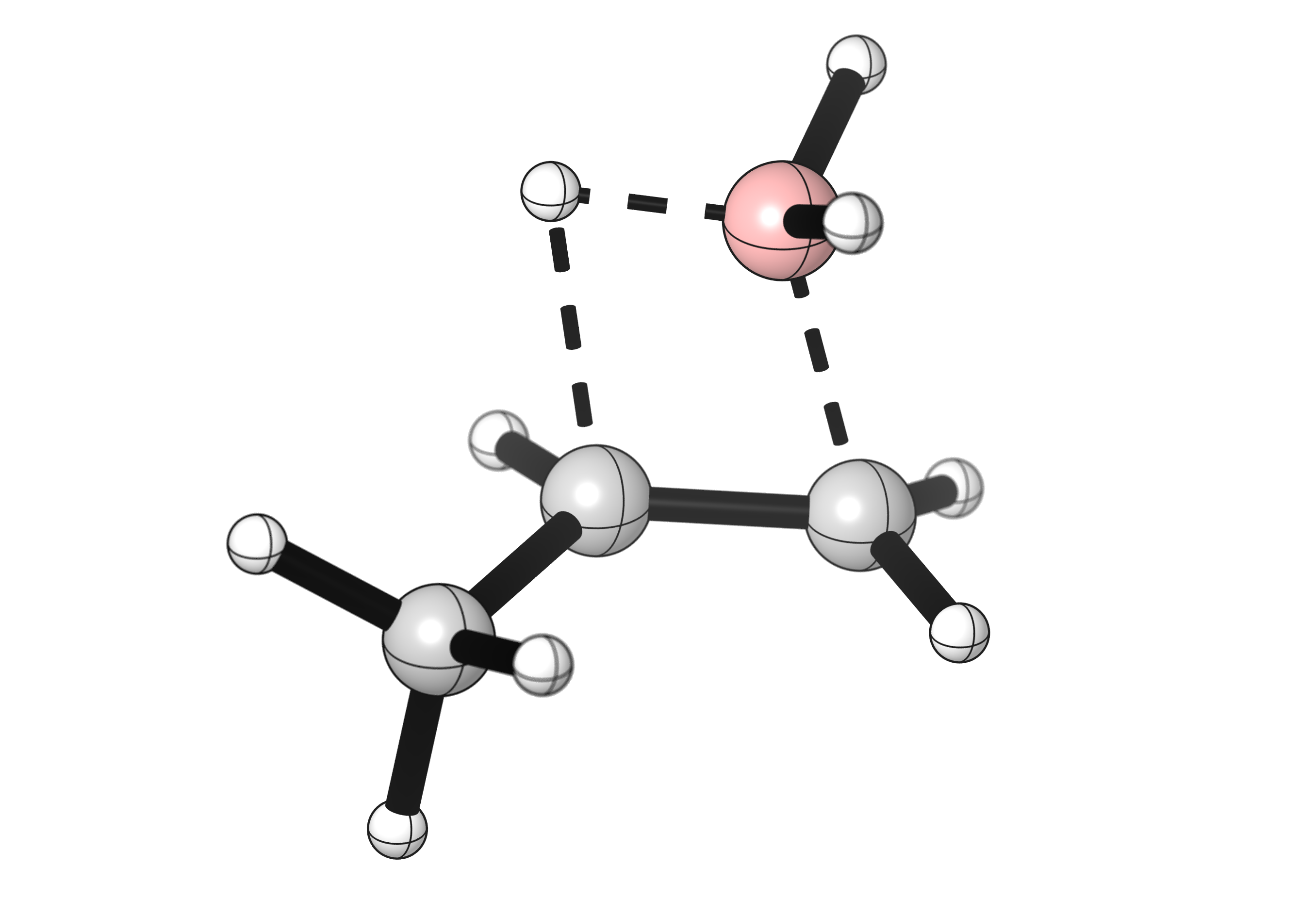
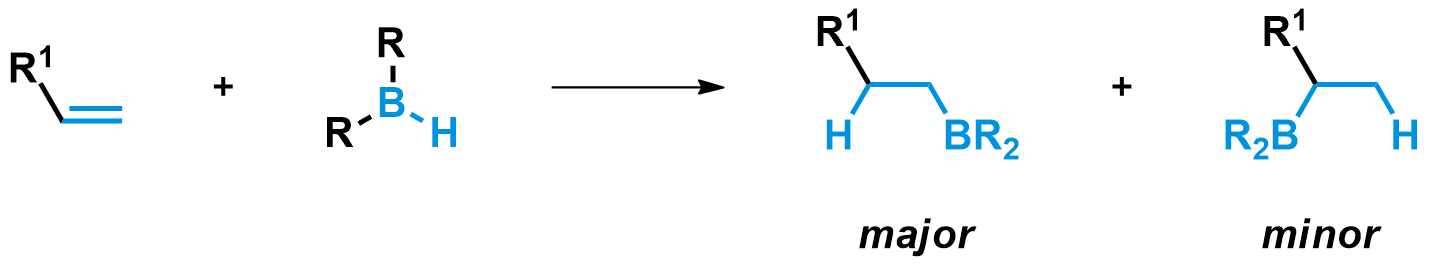
With most boranes, the hydroboration reaction is regioselective. The product with the borane attached on the least-substituted alkene carbon is formed preferentially. This is why after oxidation you can obtain the terminal alcohol as the major product.
Hydrogen peroxide oxidation
Boranes can be useful products themselves. However, in many cases the hydroboration is followed by an oxidation to obtain the terminal alcohol as product. The most common reagent combination to perform this oxidation is sodium hydroxide (NaOH) with hydrogen peroxide (H2O2). They generate a strong nucleophile, the hydroperoxide anion, which quickly binds to the electrophilic boron of the borane.
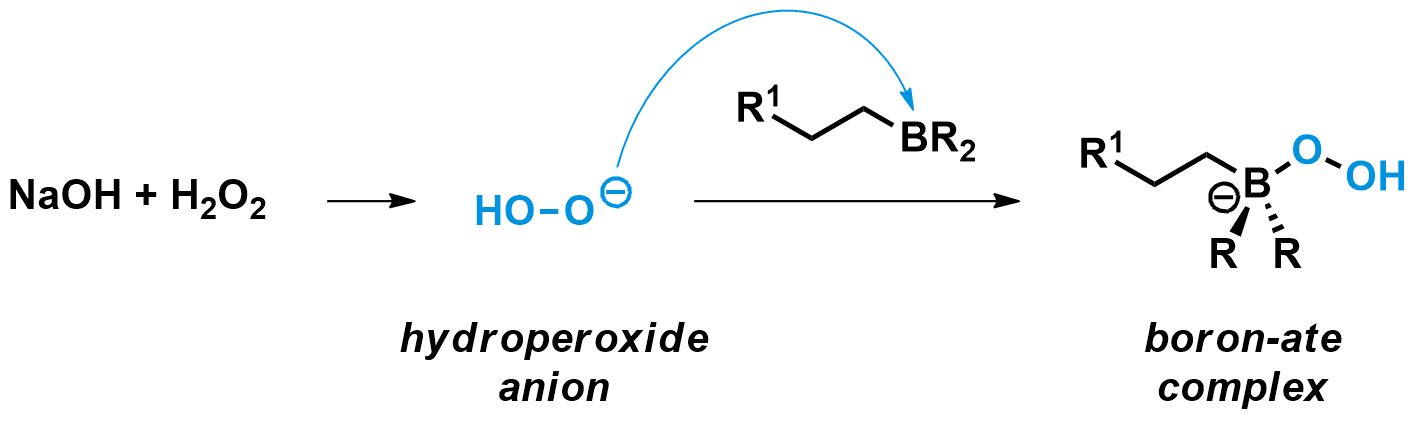
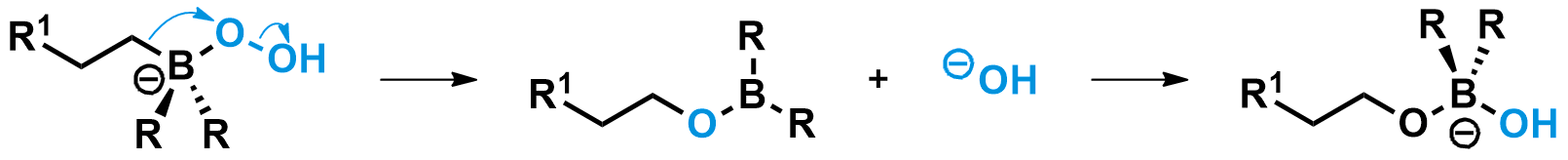
The boron-ate complex then undergoes a 1,2-shift, a mechanism unique to anionic boron compounds. During this step, the carbon group on boron migrates to the oxygen bound to it, simultaneously breaking the weak O-O bond and expelling a hydroxide leaving group. This shift is stereospecific, meaning the carbon group migrates without any changes to its configuration.

Download the data
- Intrinsic reaction coordinate (IRC) file for the anti-Markovnikov hydroboration of propene by BH3 (.xyz)
- IRC file for the Markovnikov (internal) hydroboration of propene by BH3 (.xyz)
- IRC file for the 1,2-rearrangement of hydroperoxyborate (.xyz)
Animations created by Sean Larmore.
