Rotation of the groups around a single bond can lead to conformations, which represent different three-dimensional shapes available to a single molecule. The simplest system to learn about the available conformations of alkanes is butane.
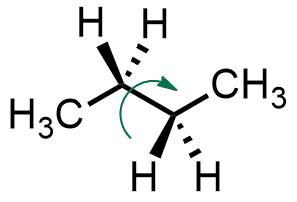
Conformations are often represented as Newman projections, looking through the central single bond of butane. From that point of view, the groups on the two central carbons can either be eclipsed or staggered. For butane, the most stable conformation is called anti (A). It is when the two methyl groups are at a 180° angle from each other when looking through the bond between the two central carbons. Subsequent rotations of 60° around that bond lead to further conformations. First, two mirror-image anticlinal (B) conformations are possible, each leading to a gauche (C) conformation. Another rotation gives the least stable conformation, called synperiplanar (D).
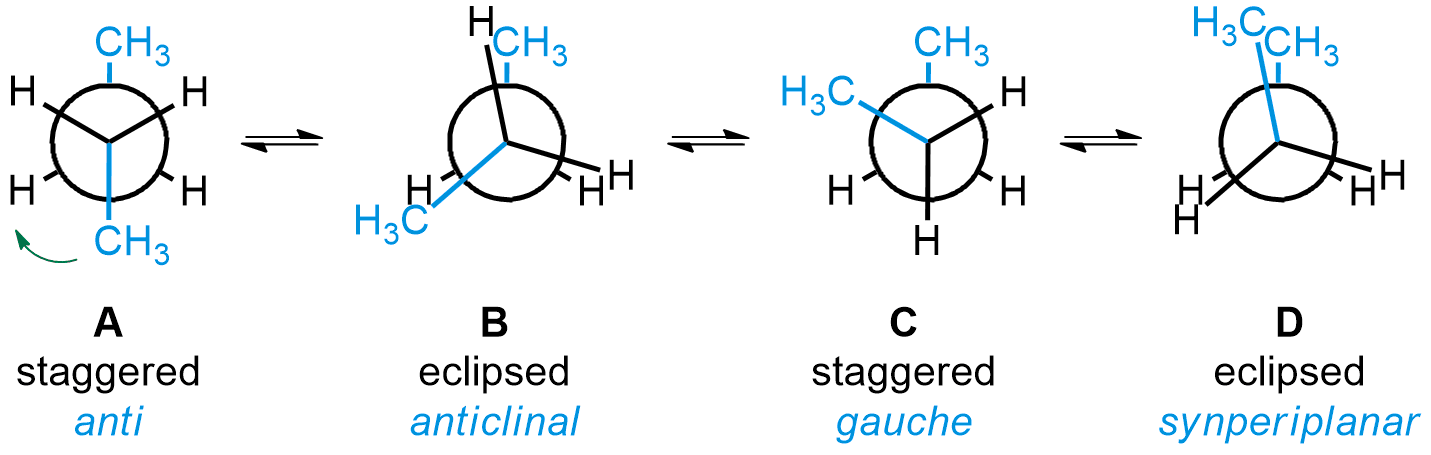
The relative stability of each conformation can be visualized by graph of relative energy as a function of dihedral angle (see below). Try to locate each conformation in the animation below, and notice how the shape of the molecule changes. Note that staggered conformations are minimum energy (stable) structures, while eclipsed conformations are maximum energy (transition) structures. Click the “Manual Animation” option to go through the butane conformations at your own pace, and remember to rotate and zoom on the animation to compare the Newman projection to the 3D structure.
Download the data
- Dihedral angle scan of butane (.xyz)
Animations created by Pier Alexandre Champagne.
