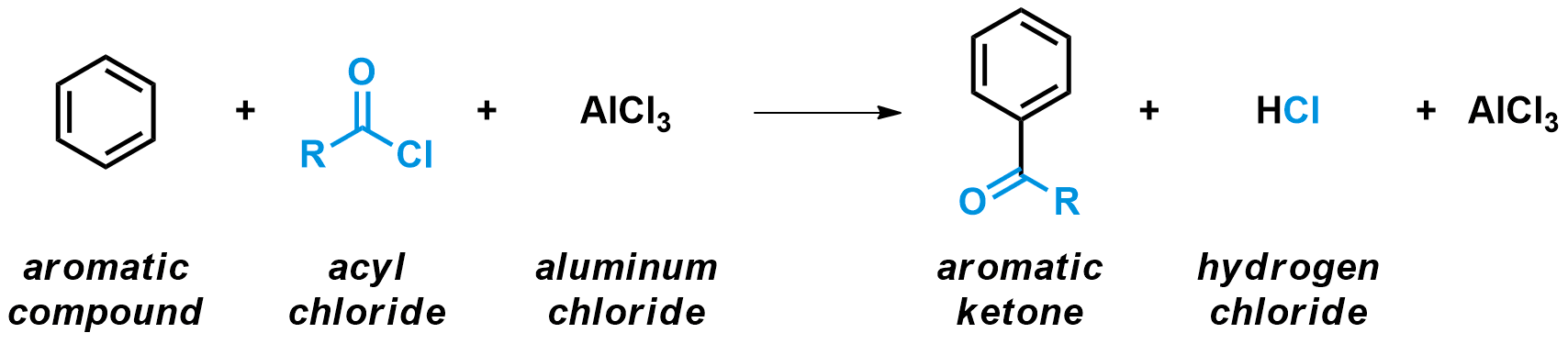
The Friedel-Crafts acylation is a specific example of electrophilic aromatic substitutions (also written SEAr). The key step for these types of reactions is always the attack of an aromatic ring onto a very electrophilic species. In the case of the Friedel-Crafts acylation, an aromatic compound reacts with an acid (or acyl) chloride in the presence of a strong Lewis acid catalyst, usually AlCl3. The products are an aromatic ketone and HCl. In the process, an aromatic hydrogen is substituted by a acyl group.

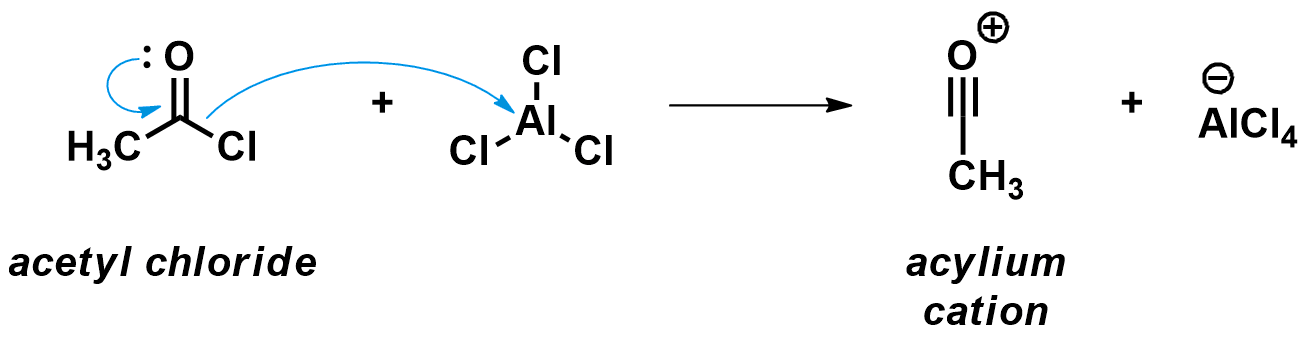
First step: Formation of the strong acylium electrophile
As aromatic molecules are very stable, they only react with strong electrophiles. Consequently, the first step of the Friedel-Crafts acylation is the formation of a very electrophilic intermediate, the acylium cation. This cation is formed when the chlorine atom of the acyl chloride binds to the strong Lewis acidic AlCl3, prompting the C-Cl bond to break. Acetyl chloride is a typical substrate for such reactions.
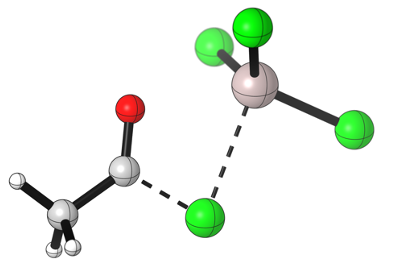
Notice how the acylium takes a linear geometry as the central carbon atom goes from an sp2 to an sp hydridization.
Second step: Attack of the aromatic on the acylium cation
Next, an aromatic compound like benzene acts as a nucleophile and attacks the highly electrophilic acylium carbon atom. A new C-C is formed, and in the process the aromaticity of the benzene ring is broken. This leads to a high energy species known as the Wheland intermediate, where the positive charge is delocalized over five carbons.
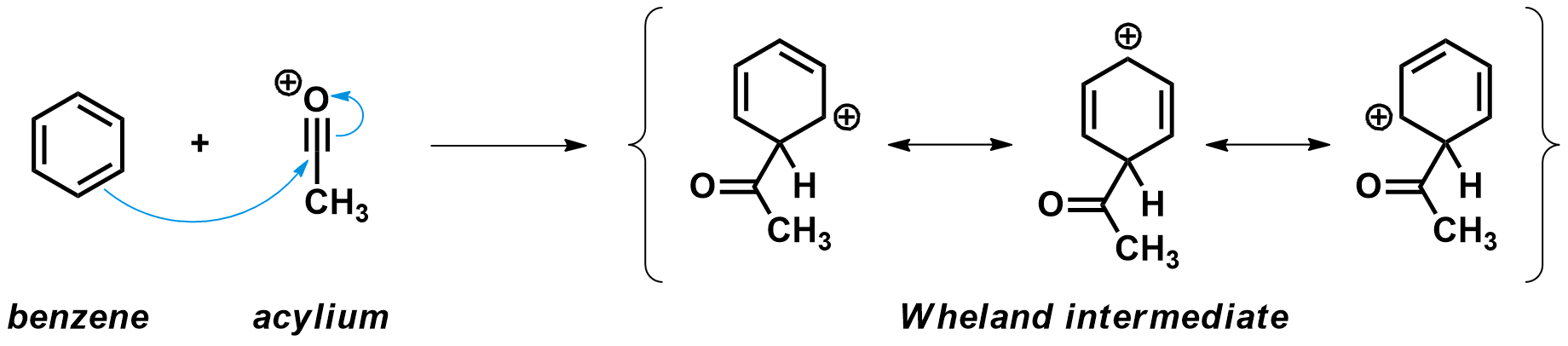
A few details to notice:
- The acylium cation approaches the aromatic compound from a 109.5 ° angle compared to the plane of the ring. This is where the necessary π molecular orbital is located.
- While the new C-C bond is formed, the hybridizations of both carbons change. The aromatic carbon moves from sp2 to sp3 hybridization and adopts a tetrahedral geometry. The acyl carbon adopts goes from sp to sp2 hybridization and adopts a trigonal planar geometry.
Third and final step: Deprotonation of the Wheland intermediate
The final step in a Friedel-Crafts acylation is the deprotonation of the Wheland intermediate. During that step, the aromaticity of the aromatic ring is restored and the acetophenone product is formed. The Wheland intermediate is very acidic; any sightly basic compound in the reaction mixture can perform this deprotonation step. Conveniently, the AlCl4– anion which was produced during the first step of the reaction is an good base. Upon deprotonation, HCl is formed and the AlCl3 catalyst is regenerated.
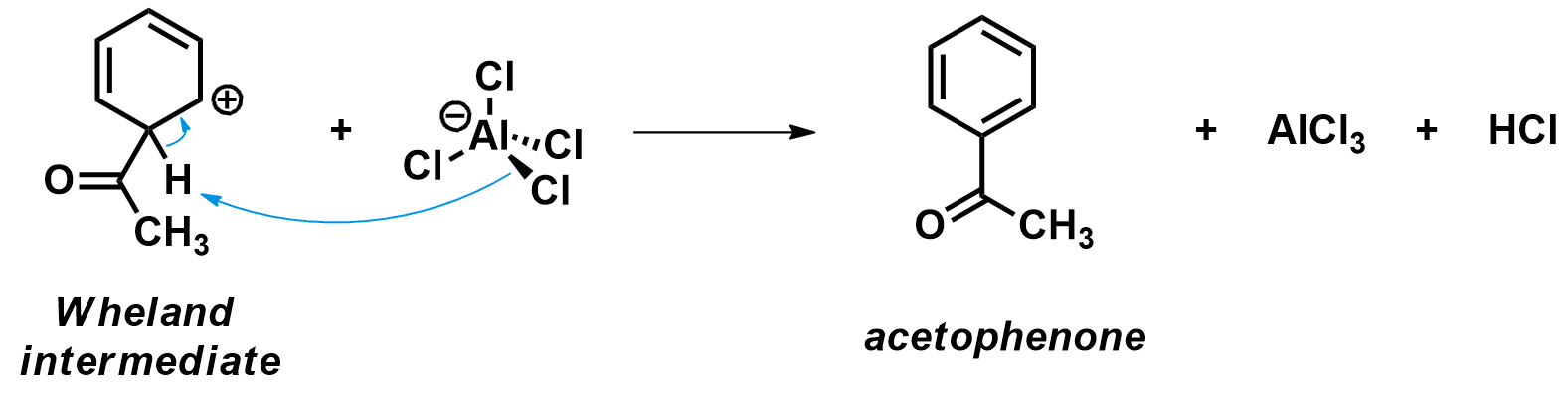
Notice how both the aluminum atom and the carbon atom move toward their regular planar geometry (sp2 hybridization) as the Wheland intermediate is deprotonated.

Download the data
- Intrinsic reaction coordinate (IRC) file for the acylium cation formation. (.xyz)
- IRC file for the acylium attack on benzene. (.xyz)
- IRC file for the deprotonation step. (.xyz)
Animations created by Floris Buttard.
