The basics
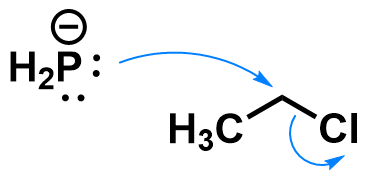
Substitution reactions are a type of organic transformations where one group on an atom (the leaving group) is replaced by another group (the nucleophile). Substitution reactions can occur via a variety of mechanisms, which depend on the substrate, nucleophile, solvent, etc. A classic example of nucleophilic substitution happens on haloalkanes like chloroethane, in the presence of strong anionic nucleophiles like phosphide.

This reaction occurs through an SN2 mechanism, which is a concerted (one-step) mechanism. The nucleophile forms a new bond to the carbon, and simultaneously the bond to the leaving group breaks. How does it look like when a substrate undergoes an SN2 reaction?
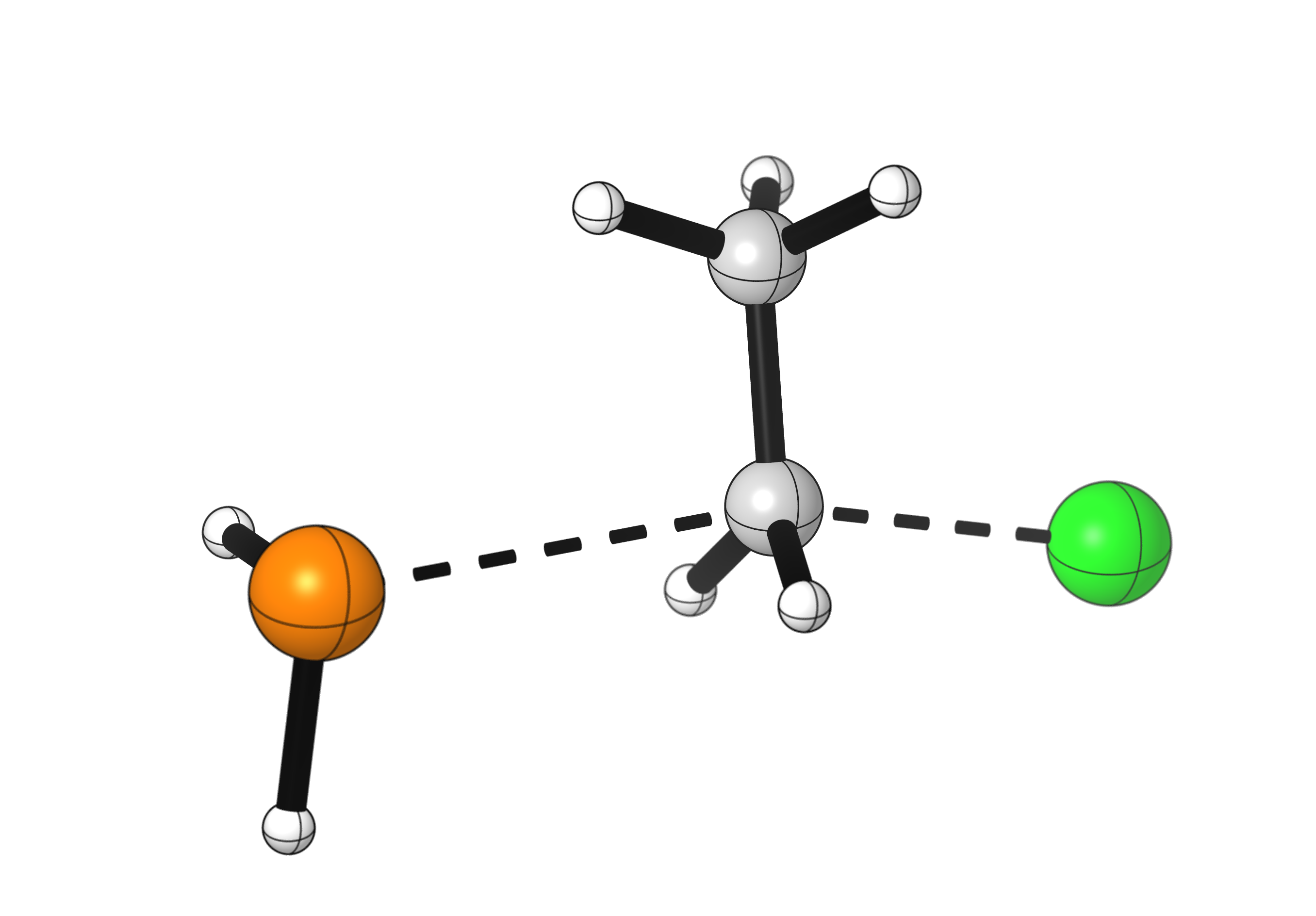
A few details to notice:
- The bond cleavage and formation happen simultaneously.
- The nucleophile approaches the carbon at a 180° angle from the leaving group. This is an orbital requirement for SN2 reactions.
- During the reaction, the configuration at the carbon atom flips. Notice how the hydrogen atoms point in a different direction after the reaction.
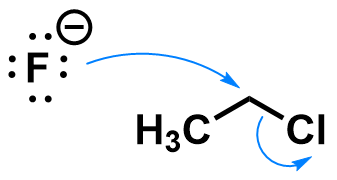
Chloroethane substitution by fluoride
A similar reaction could occur with fluoride as a nucleophile.

Fluoride forms a stronger bond to carbon than chlorine, so this reaction is very energetically favorable.
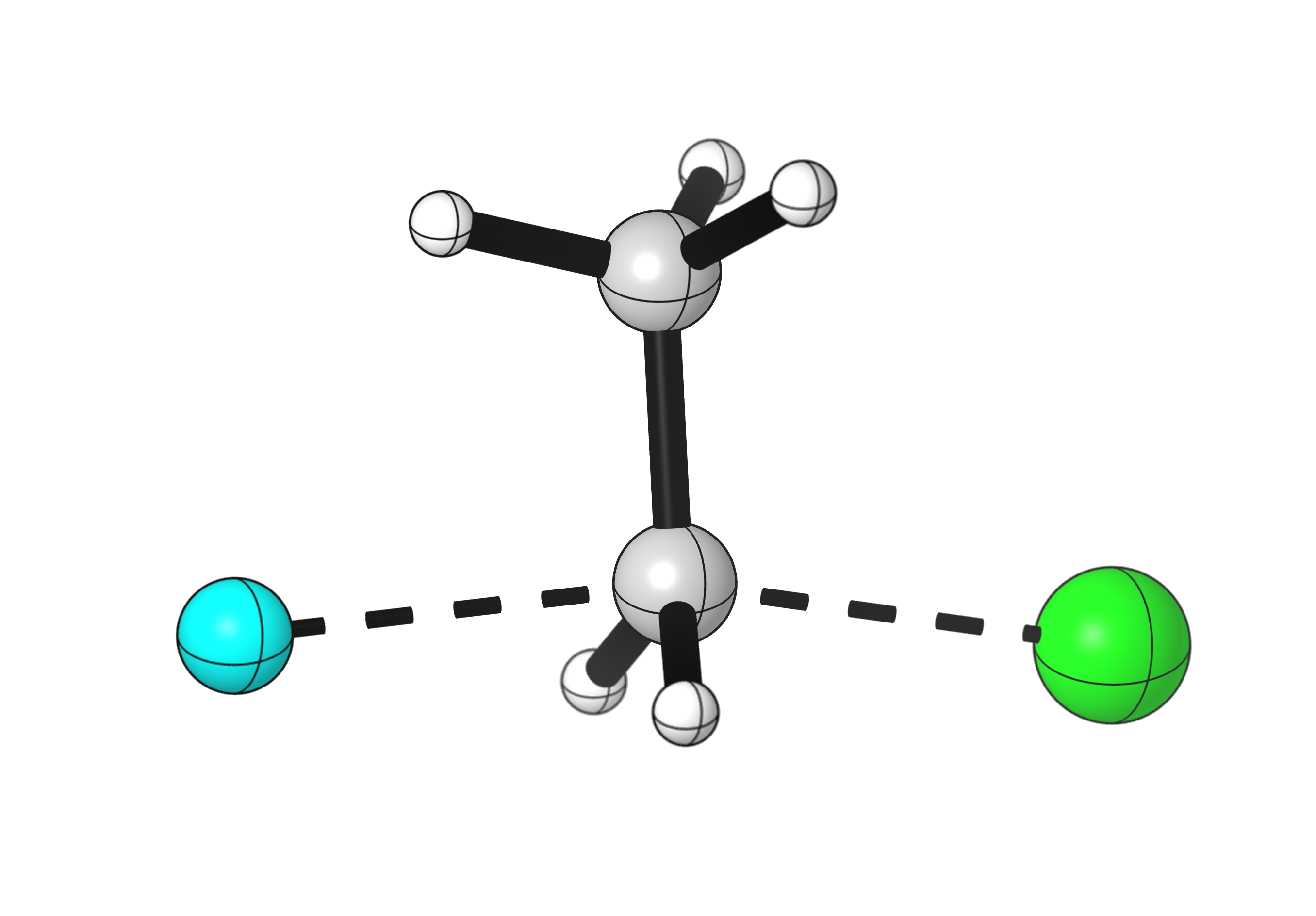
Download the data
- Intrinsic reaction coordinate (IRC) file for the substitution of chloroethane by phosphide. (.xyz)
- IRC file for the substitution of chloroethane by fluoride. (.xyz)
Animations created by Joie Kelly, Rebecca Zaki, Sean Larmore, and Floris Buttard.
