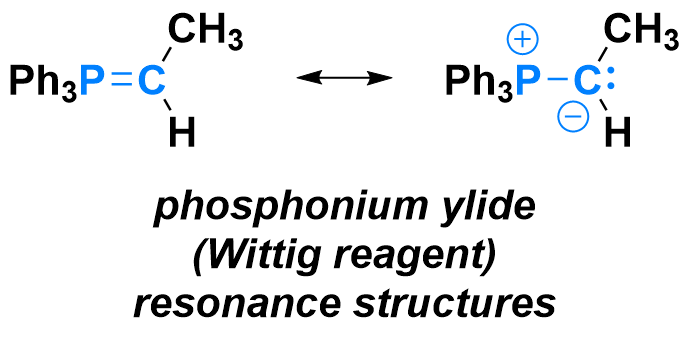
The Wittig olefination is the transformation of a ketone or aldehyde into an alkene, under the action of a phosphonium ylide (right), so-called Wittig reagent. Phosphonium ylides can be made by deprotonation of an alkyl phosphonium salt, and are characterized by a short P-C bond connecting a positively-charged phosphorus with a negatively-charged carbon. As hinted by their resonance structures, phosphonium ylides are nucleophilic at the carbon atom.
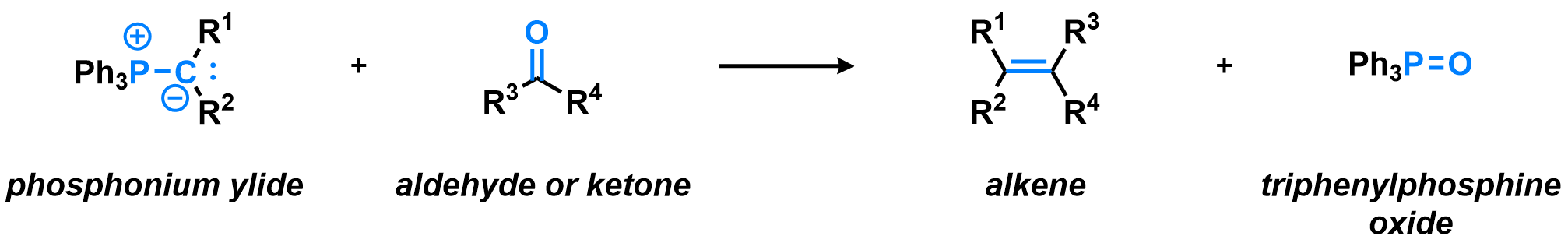
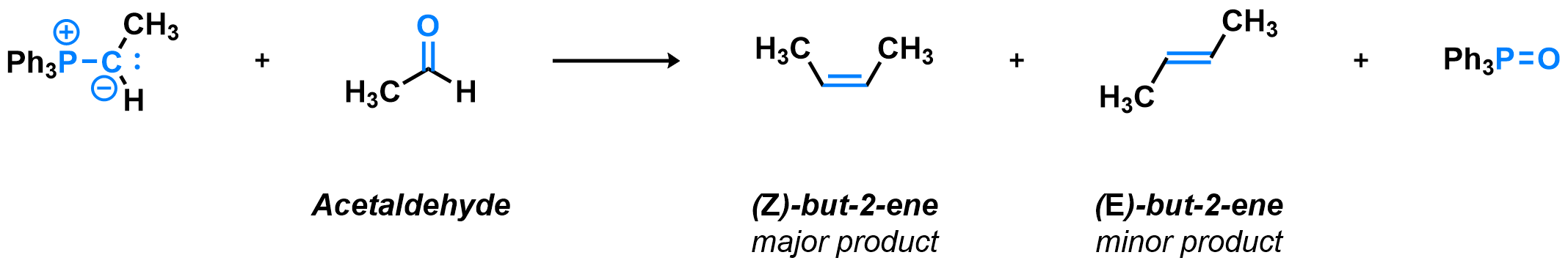
In most Wittig reactions, both the (E) and (Z) isomers of the alkene product can be formed. Taking the simple example of acetaldehyde reacting with a triphenylphosphonium ylide bearing a single methyl substituent, or other unsubstituted ylides bearing alkyl groups, the (Z)-alkene (cis) is formed in good selectivity. For substituted ylides, bearing aryl or electron-withdrawing substituents, the selectivity is different change and the mechanistic explanation for this behavior is still not fully defined. Many variants of the Wittig olefination exist, using different reagents and providing different selectivity, for example the Horner-Wadsworth-Emmons reaction or Julia olefination, to name a few.
First step: Formation of the oxaphosphetane
In the first step of the Wittig olefination, the nucleophilic carbon of the ylide attacks the electrophilic carbon of the carbonyl substrate, forming a betaine (zwitterion). This structure quickly collapses to the oxaphosphetane, an intermediate containing 4-membered heterocycle involving a P-O bond. The intermediacy of the betaine is still debated, and it is thought that in most cases the oxaphosphetane is the first stable intermediate formed in this reaction.
This is the case for the example below. Notice how the C-C bond forms much earlier than the P-O bond, although there is no minimum energy structure without that P-O bond. Attack of the formation of the C-C bond during this step changes the hybridization of the two reacting species from sp2 to sp3. Also notice how the final relationship between the methyl groups is determined during this step.
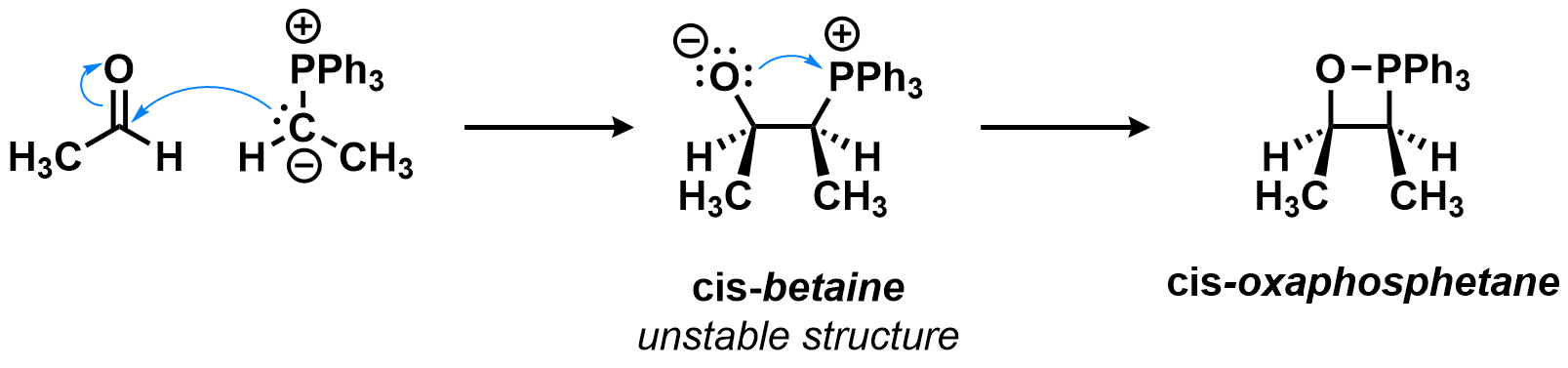
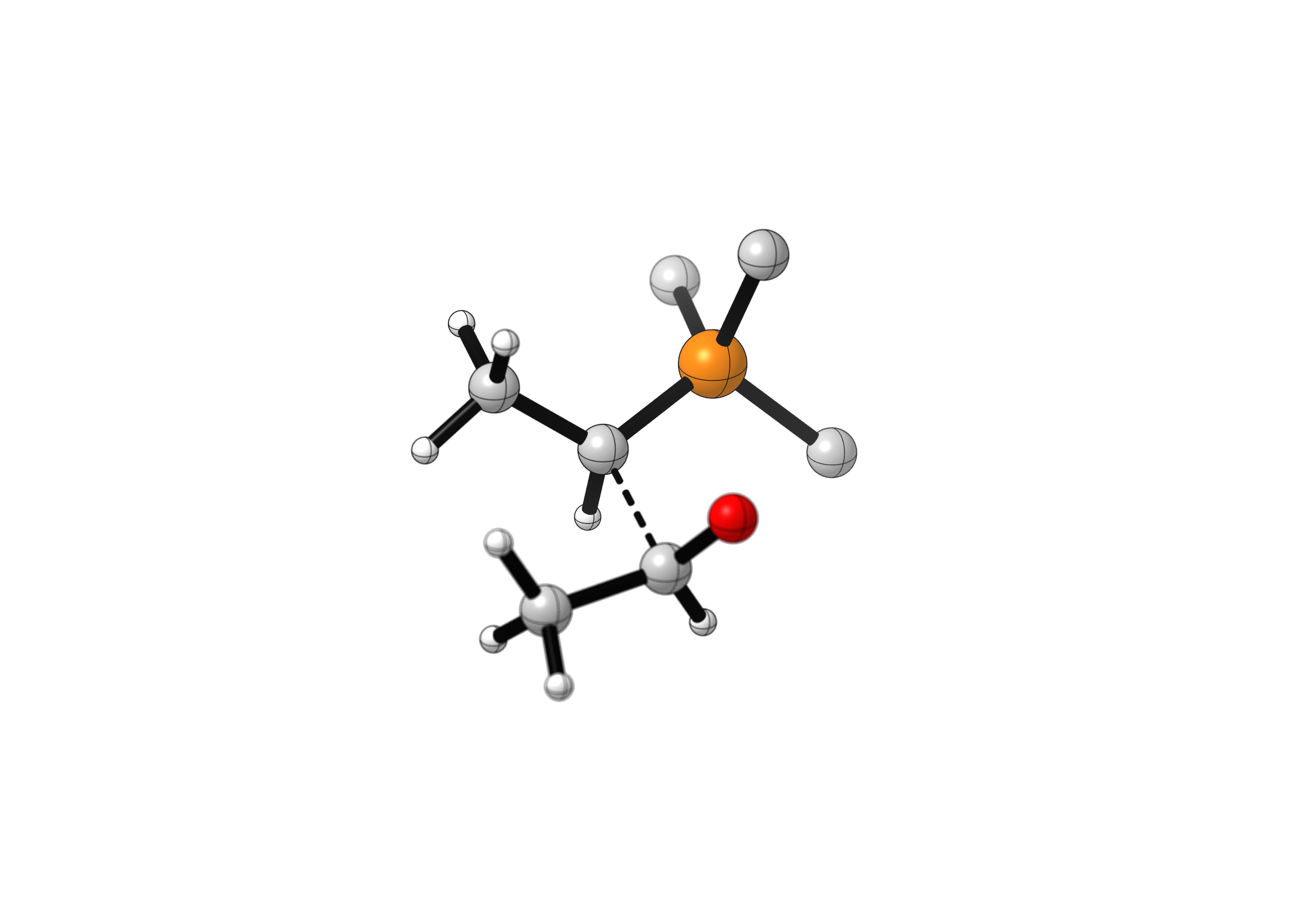
(phenyl substituents hidden)
Second step: Collapse of the oxaphosphetane
In the second step, the oxaphosphetane collapses, yielding two pi-bond containing products: the desired alkene and the phosphine oxide side-product. This step is favorable for two reasons: 1) the angle strain of the 4-membered heterocycle, and 2) the strength of the P=O double bond, which serves as a thermodynamic driving force in this reaction.
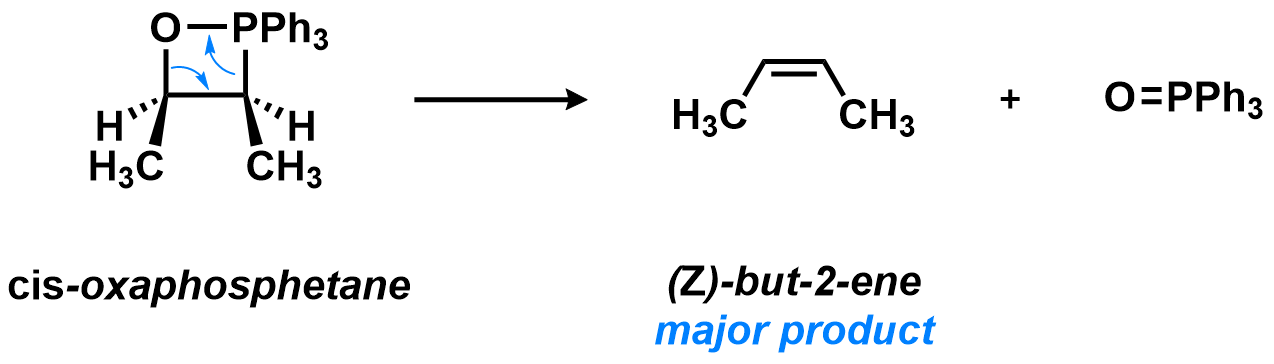
The arrows used to show this step might seem complicated, but they simply indicate that two bonds break (those at the start of the arrows) and two new double bonds are formed (those at the end of the arrows). As such, the two products simply drift apart, see:
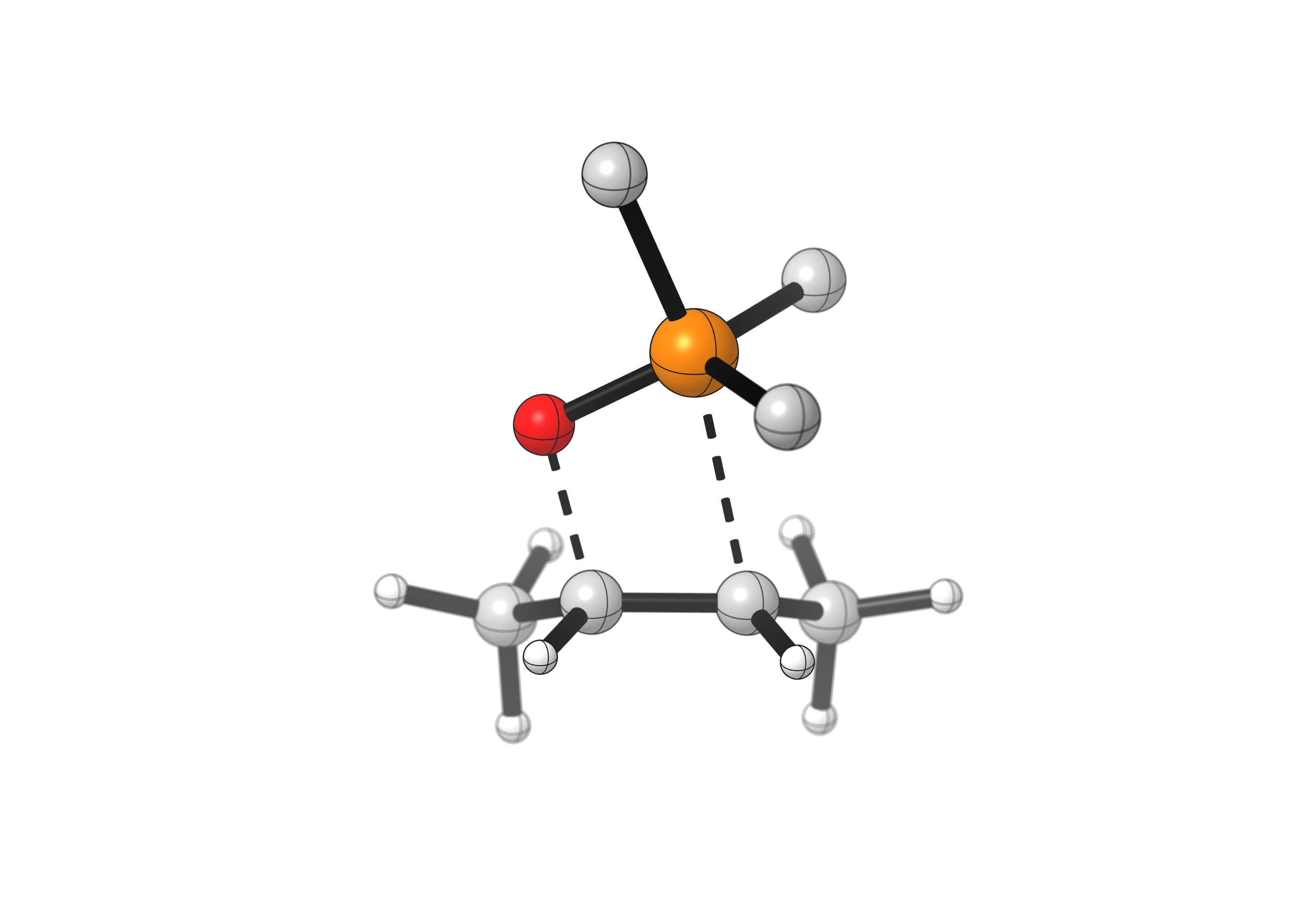
(phenyl substituents hidden)
Download the data
- Intrinsic reaction coordinate (IRC) file for ylide attack on the carbonyl. (.xyz)
- IRC file for oxaphosphetane collapse. (.xyz)
Animations created by Rebecca Zaki.
