The epoxidation of alkenes gives an oxygen-containing three-membered ring called an epoxide (IUPAC: oxirane). For this reaction, an alkene is reacted with an epoxidation reagent, usually a peroxyacid. Peroxyacids are derivatives of carboxylic acids that contain an additional O-O bond. The peroxyacid reagent forms an acid as by-product, while the epoxide is formed.
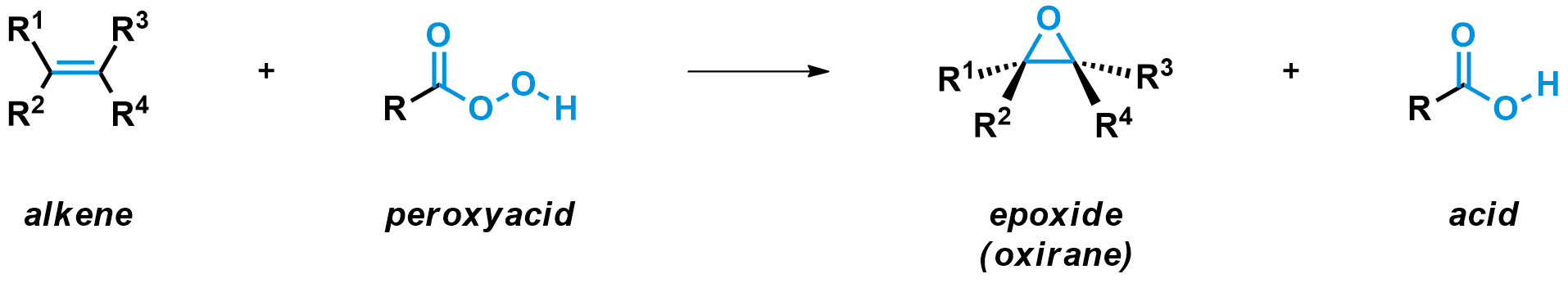
The most common peroxyacid used for the epoxidation of alkenes (like propene) is meta-chloroperoxybenzoic acid, or mCPBA. The mechanism for the reaction of mCPBA with alkenes is complex. As the alkene approaches the peroxyacid, two new C-O bonds are formed. At the same time, the O-O bond is broken, pushing the carbonyl to attack the hydrogen, which leaves its electrons to the oxygen. Five different bonds are formed and broken in one concerted step, making it a rather unique mechanism. Usually no more than three electron pairs are moved during a single chemical step.
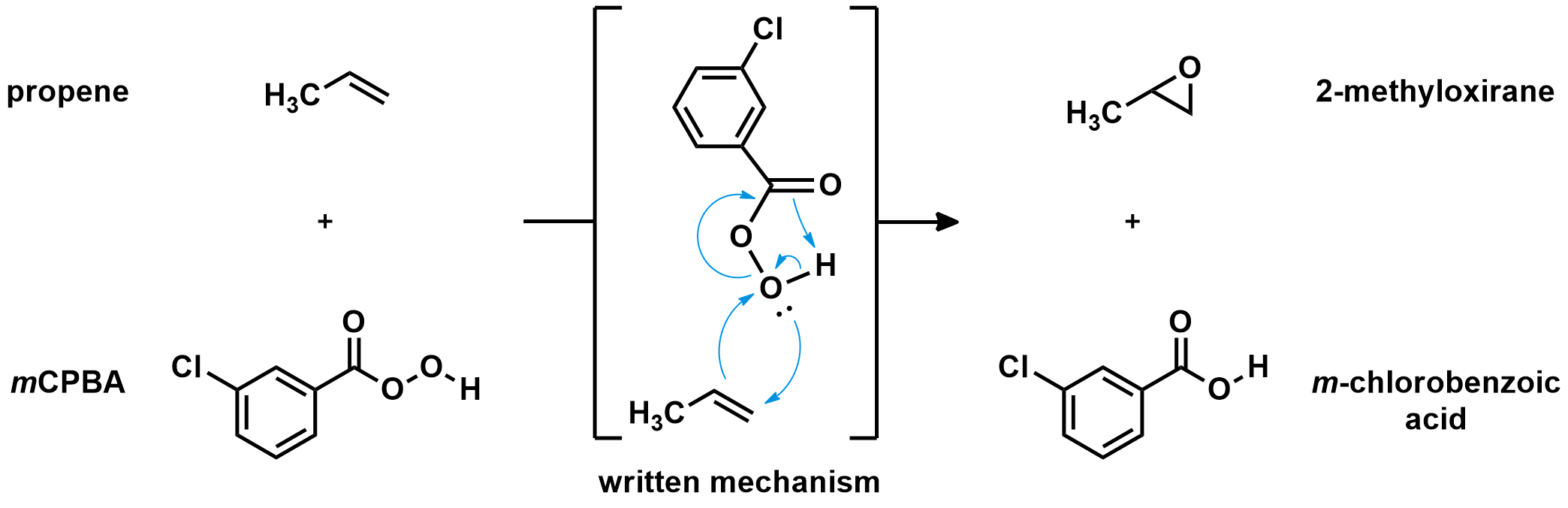
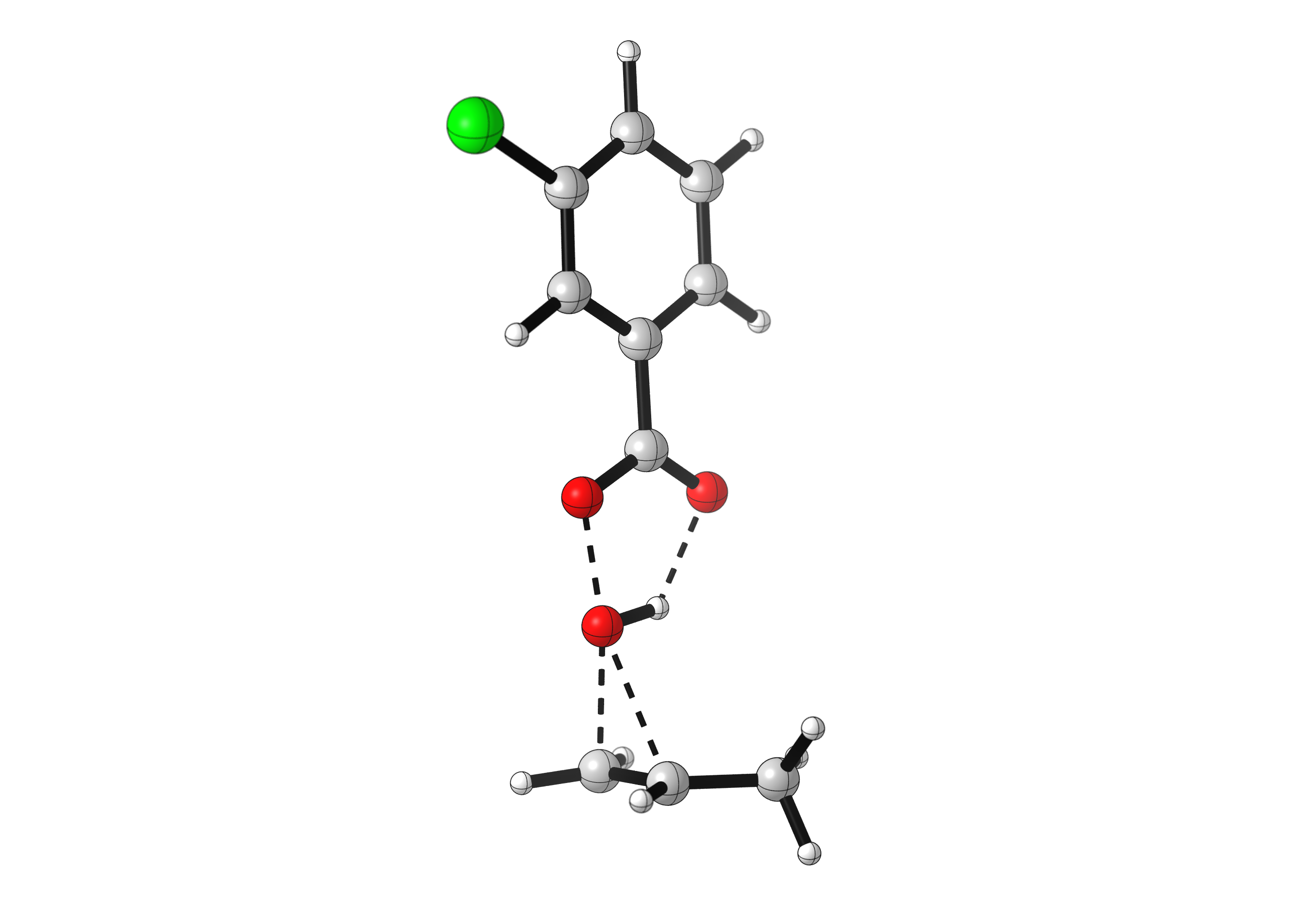
As many bonds are formed and broken simultaneously, the epoxidation reaction has strict orbital requirements. The peroxyacid has to adopt a planar arrangement, and approach the alkene perpendicular to the C=C bond. See for yourself:
Epoxide hydrolysis to form diols
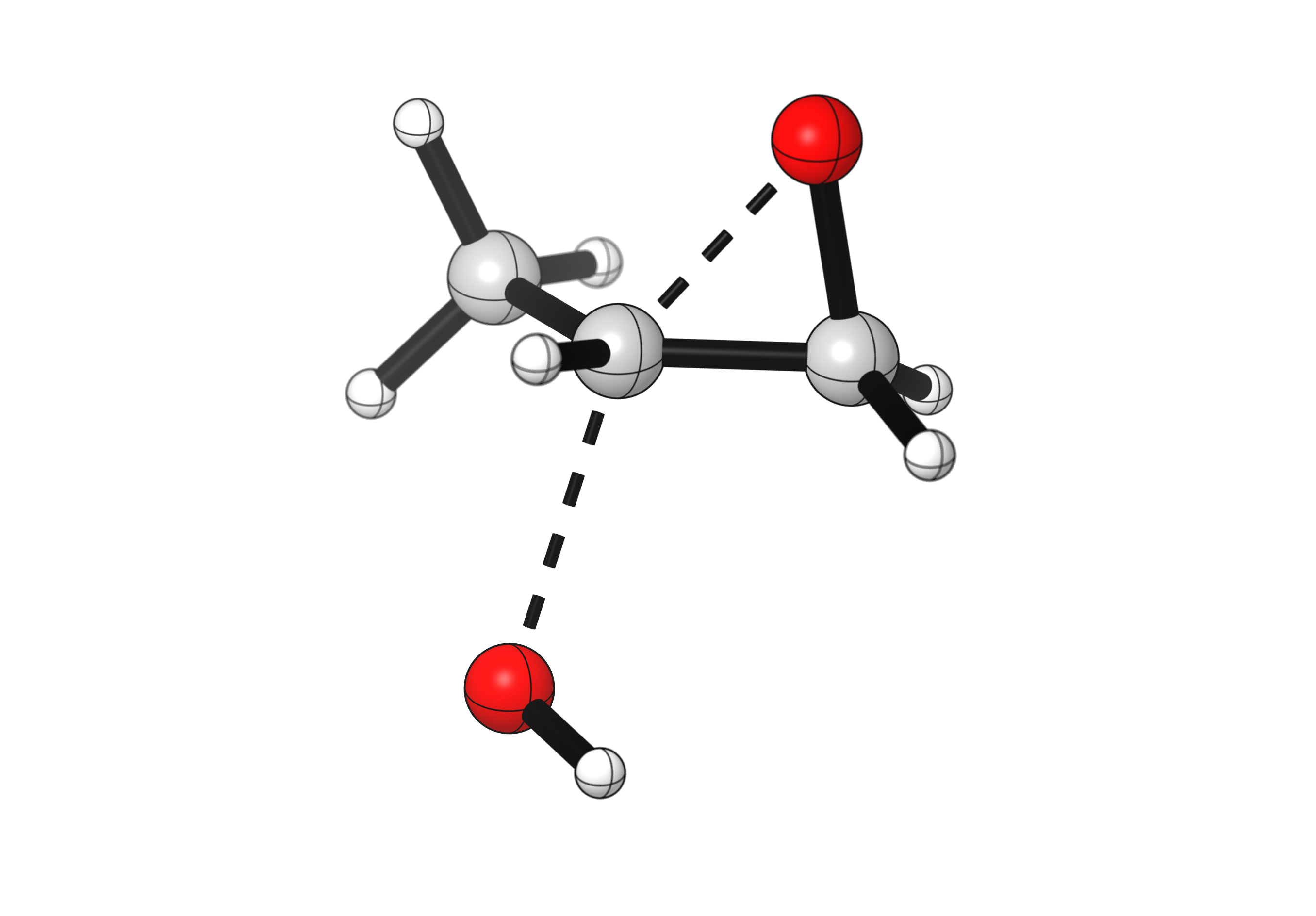
Epoxides are good electrophiles for nucleophilic opening. A common reaction of epoxides is hydrolysis to form diols. Under basic conditions, the hydroxide anion (HO–) will preferentially attack the most unhindered carbon of the epoxide. For a monosubstituted epoxide like 2-methyloxirane, terminal and internal attacks will result in the same final product, but that is not the case for all epoxides. Upon opening, the alkoxide will be protonated by the solvent, generating the diol product.

These nucleophilic openings of epoxides resemble SN2 reactions, as the HO– approaches from the back of the breaking C-O bond. Notice below how the carbon undergoing substitution inverts its configuration during the reaction, while the other carbon stays in its sp3 hybridization throughout the process.

Download the data
- Intrinsic reaction coordinate (IRC) file for the epoxidation of propene by mCPBA (.xyz)
- IRC file for the epoxide opening at the terminal position (.xyz)
- IRC file for the epoxide opening at the internal position (.xyz)
Animations created by Rebecca Zaki.
