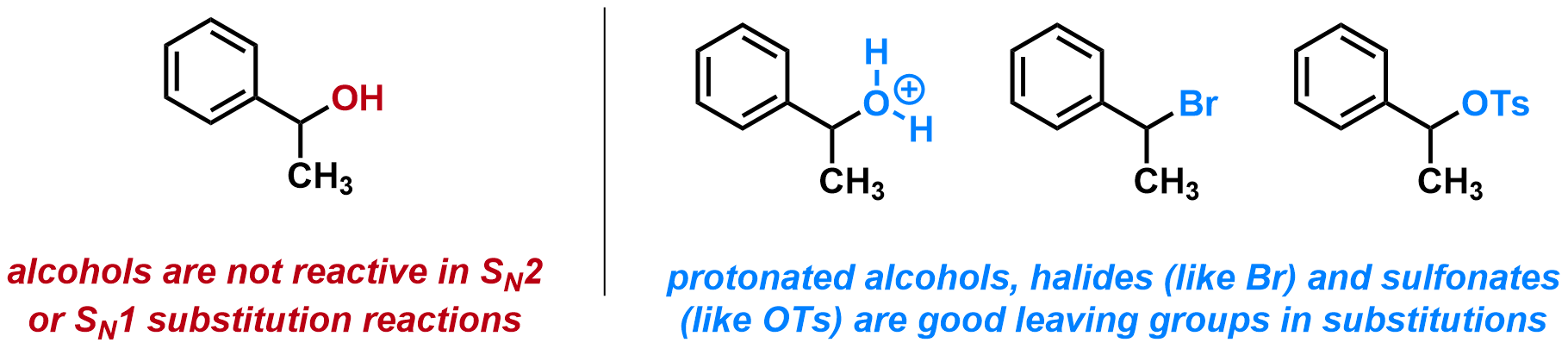
The key to substitution and elimination reactions is to have a good leaving group, meaning an electronegative atom or functional group that is weakly attached to carbon and that can accept the incoming electrons. Alcohols are not leaving groups, however they can be protonated, or transformed into halides or sulfonates, all of which are good leaving groups.
For a given substrate, two main substitution mechanisms are possible, SN1 and SN2. As the SN1 mechanism involves a two-step pathway with an intermediate carbocation, it becomes preferred for substrates that can form stabilized carbocations, and when the nucleophile is poor so that the bimolecular SN2 mechanism is difficult. One example would be the reaction of secondary benzylic alcohols with a thiol, in acidic conditions.
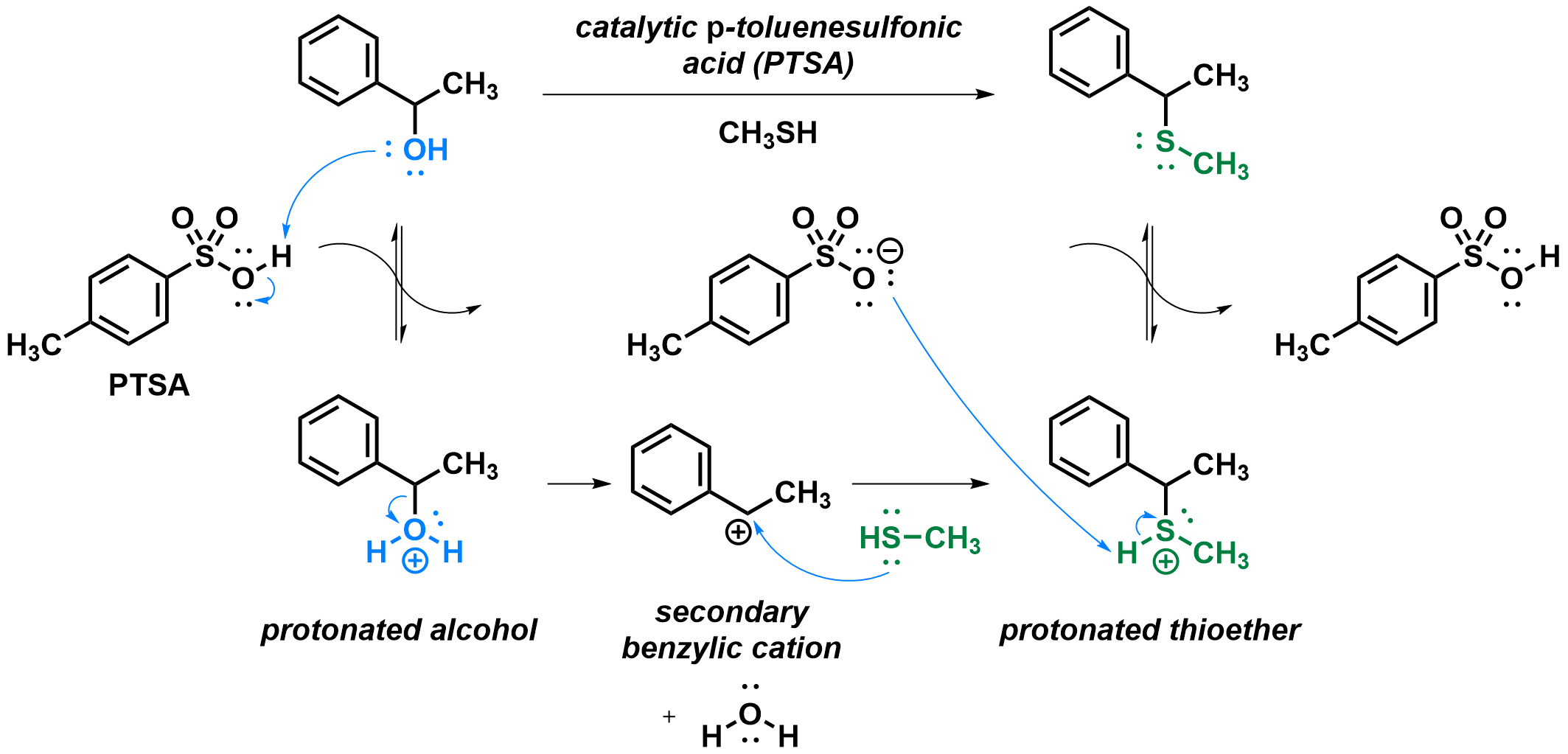
Formation of the carbocation by loss of water
Capture of the carbocation by the nucleophile
First, the alcohol must be protonated by the acid catalyst before any substitution can occur. This step is under acid-base equilibrium as the pKa of the PTSA (-2.8) and that of protonated alcohols (around -2) are similar. Then, the leaving group (in our case, a water molecule) drifts apart from the carbon, leaving behind a carbocation as you can see in the animation below. Notice how the hybridization of the carbon atom changes from sp3 (tetrahedral) to sp2 (trigonal planar) as the C-O bond is broken. The nucleophile (in our case methanethiol) can then approach the carbocation and use a sulfur lone pair to create a new C-S bond, turning the carbon tetrahedral again. Finally, the protonated thioether can give its proton back to the catalyst, completing the reaction. As you can see, the formation and capture of the cation are the reverse steps of one another.
Download the data
- Intrinsic reaction coordinate (IRC) file for the loss of water from the protonated alcohol (.xyz)
- IRC file for the attack of methanethiol on the cation (.xyz)
Animation created and shared by Benjamin Mackenzie and Leanne Racicot (University of Waterloo, https://uwaterloo.ca/racicot-organic-chemistry-lab/).
