One important feature of SN2 reactions is that they are stereospecific, meaning that when the substitution happens on a stereocenter, only one stereoisomer of the product is formed for every stereoisomer of the reactant. Specifically, due to the approach of the nucleophile during the SN2 mechanism, the configuration on the stereocenter is inverted during the reaction.
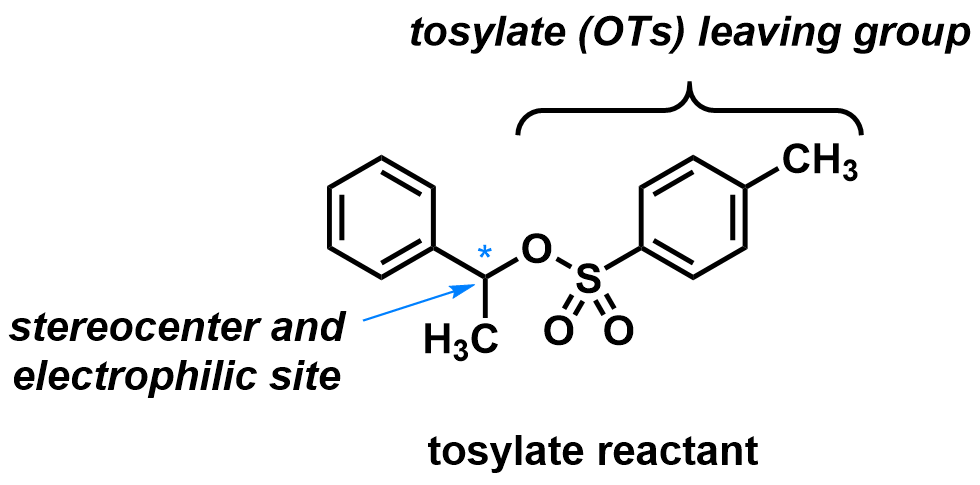
As an example, the secondary benzylic tosylate (reactant, shown right) is chiral and the stereocenter is the point of substitution. With a strong nucleophile like methane thiolate (CH3S–) in a polar aprotic solvent like acetonitrile (CH3CN), an SN2 mechanism is expected. Therefore, for each enantiomer of the reactant, a different enantiomer of the product will be formed.
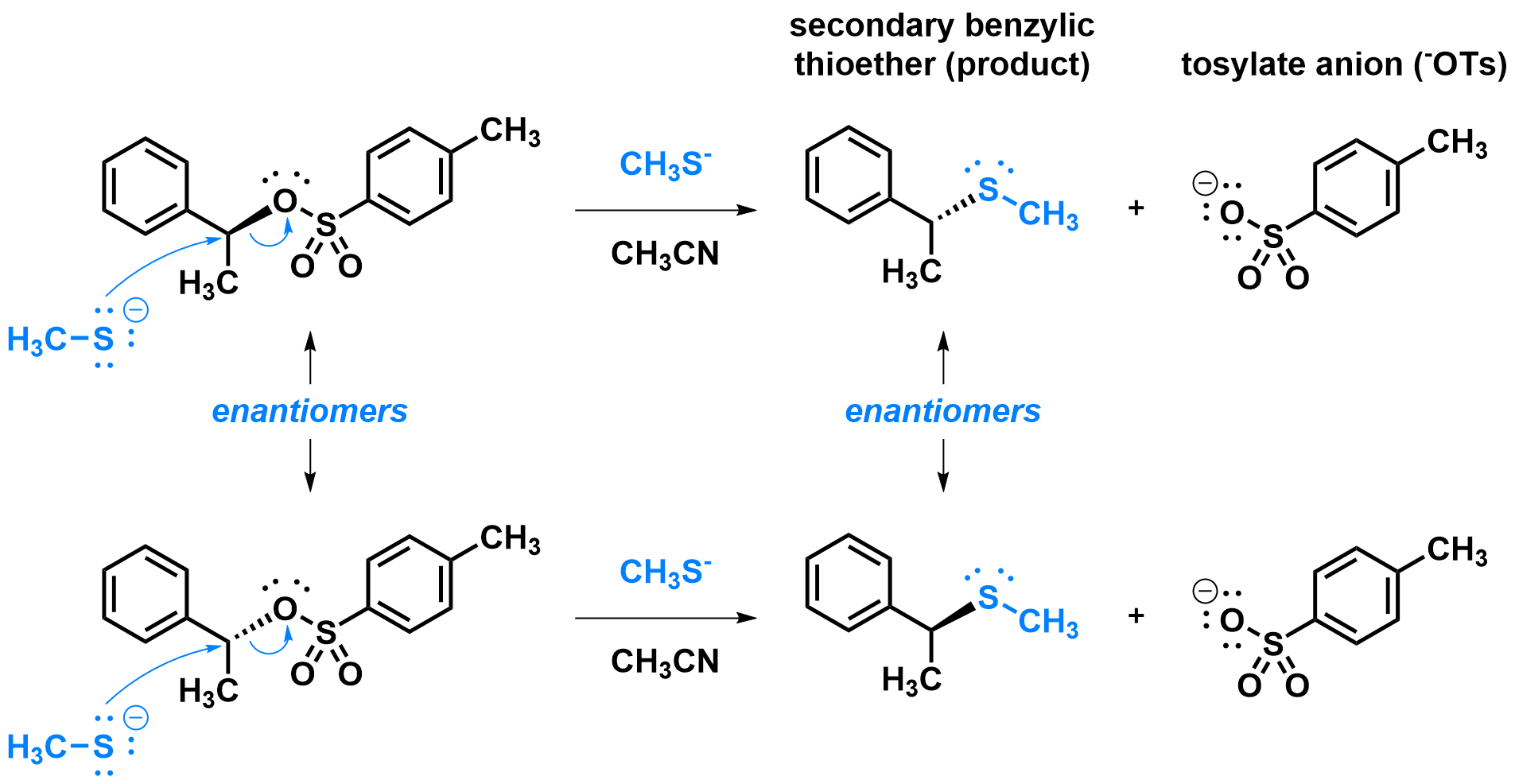
Which of the two reactions above is shown in the animation below?
Download the data
- Intrinsic reaction coordinate (IRC) file for the stereospecific SN2 (.xyz)
Animation created and shared by Benjamin Mackenzie and Leanne Racicot (University of Waterloo, https://uwaterloo.ca/racicot-organic-chemistry-lab/).
